Overview:
Perhaps the most fundamental challenge faced by the adaptive immune system is how to distinguish foreign pathogens from normal host stimuli. Our lab is interested in the biochemical and transcriptional strategies employed by lymphocytes to do so; we aim to identify the mechanisms that enable T and B cells to discriminate self from foreign.
(a) The lab seeks to define the rules and mechanisms that govern B cell responses to specific features of self and foreign antigens, including antigen affinity, valency, biophysical structure, and co-stimulatory signals. Against this backdrop, we want to understand how self-reactive B cells, despite chronic antigen engagement of the B cell antigen receptor (BCR), are restrained from inappropriate activation and differentiation into antibody-secreting plasma cells. We want to define how this process is disrupted in a variety of autoimmune disease states, and how normal regulatory mechanisms (both biochemical and transcriptional) can be harnessed to restore tolerance on the one hand or optimize vaccine responses on the other.
(b) We are also interested in how developing T cells in the thymus interpret self antigen encounter in order to adopt distinct fates according to the type and intensity of signals received through the T cell antigen receptor (TCR) : deletion, diversion into regulatory lineages, or entry into the mature conventional T cell repertoire. We are also probing how thymocytes adapt and tune their signaling machinery to optimize affinity discrimination and limit inappropriate responses to self.
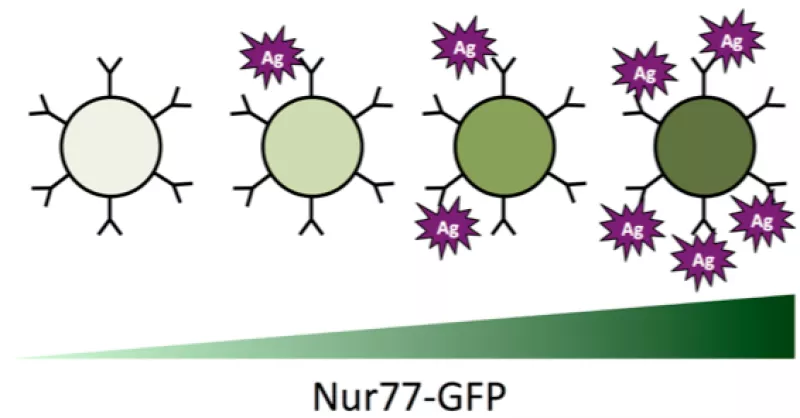
Our lab takes a range of creative approaches that span mouse genetics, cellular immunology, and genomics in order to address these questions. In particular, we have characterized and exploited novel reporter mice (NUR77-eGFP BAC transgenic) in which antigen receptor signaling drives expression of eGFP. This reporter serves as an in vivo sensor of both self and foreign antigen encounter, and serves to unmask enormous clonal heterogeneity among superficially uniform populations of lymphocytes.
Projects:
Prior work has focused on dissecting the distinct roles of the IgM and IgD B cell receptor isotypes in regulating the immune responses of self-reactive B cells. Recent projects have sought to define transcriptional mechanisms by which NUR77/Nr4a1 and related orphan nuclear hormone receptors of the NR4A family function to regulate B responses to antigen as well as how this transcriptional machinery operates during central T cell tolerance. Finally, we exploit a range of conventional and single cell approaches to study biochemical pathways that enable B cells to discriminate self and virus-like antigens.
Location:
The Zikherman Lab is located on the Parnassus Campus at UCSF. We occupy open lab space with commanding views of of Golden Gate Park, the Marin Headlands, the Presidio, the Inner Sunset, and the Pacific Ocean. We share the floor with five other labs spanning departments but with a shared focus on immunology and inflammation. The surrounding neighborhood hosts a number of nice restaurants and bars for lunch and happy hours. The UCSF Parnassus campus is located on the SF Muni N-Judah line and there are multiple UCSF shuttles that travel between different UCSF campuses across the city making it easily accessible from most areas of San Francisco. Magnificent hiking trails are just across the Golden Gate Bridge (see lab hike through Redwoods below), although some great ones are closer to home!