Regulation of B cell tolerance to self-antigens and humoral immune responses to foreign antigens by the NR4A family
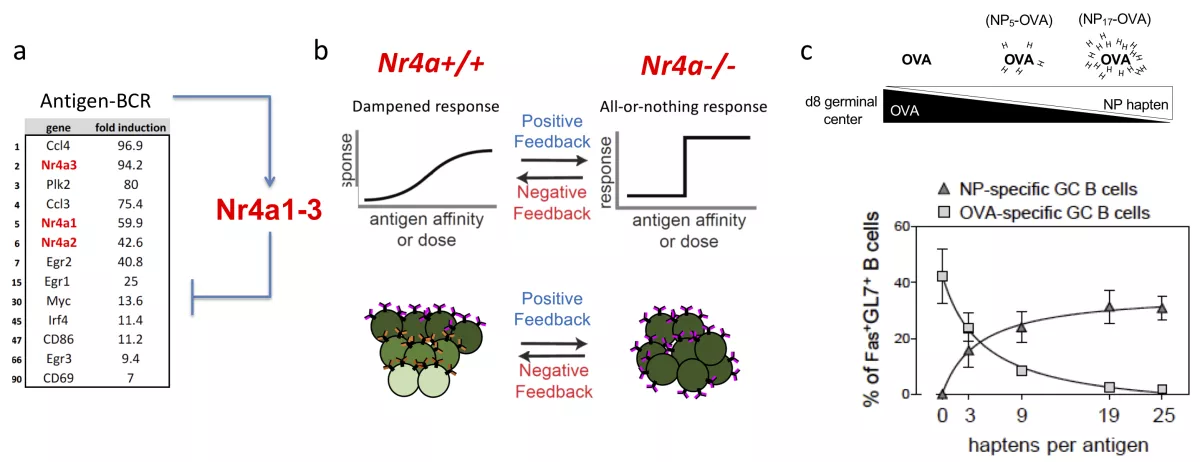
a. Nur77 (Nr4a1) and its family members encode nuclear hormone receptors that are upregulated in self-reactive B cells in response to chronic self-antigen stimulation. Our lab is taking advantage of mice with conditional and germline deficiency in Nur77/Nr4a1 and its family members to understand their function in the context of tolerance. We have identified roles for this family in limiting the survival and expansion of B cells that receive “signal 1” (antigen) in the absence of “signal 2” (co-stimulation).
b. The NR4A family of nuclear hormone receptors are upregulated not only by chronic antigen stimulation, but also by acute antigen stimulation. Here they restrain the transcription of other immediate-early genes, including those required to recruit T cell help. We seek to understand the molecular basis and functional implications of this new regulatory network.
c. Upon antigen encounter, up to 100 different B cell clones can seed an individual GC. This clonal diversity is considered essential for driving competition and optimal affinity maturation as the GC reaction progresses. We hypothesize that a negative feedback loop operates downstream of antigen receptor signaling in B cells in order to promote clonal diversity in the early GC, and prevent collapse of such diversity prematurely as the GC evolves. We postulate further that the NR4A family may mediate one such negative feedback loop, and have developed reductionist approaches to test this hypothesis and to study how clonal diversity is balanced against clonal immunodominance.
Role of antigen affinity and density in optimal vaccine design
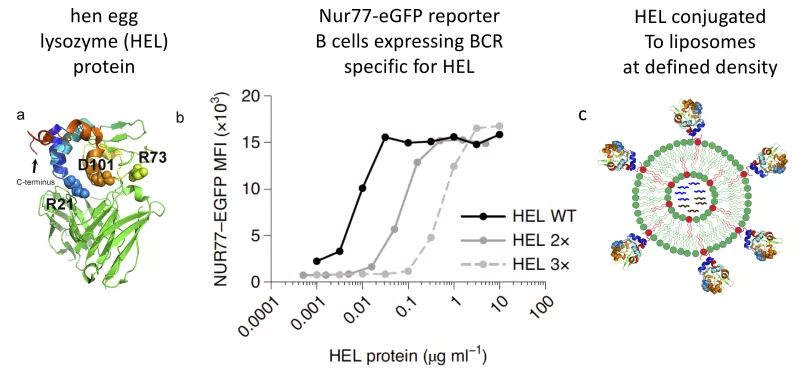
Although most studies of humoral immune responses in mice rely upon model hapten-protein antigens, bona fide pathogens such as viruses display multi-valent antigens on their surface at precise densities across a broad range. We hypothesize that B cells have evolved the capacity to recognize essential features of viruses as “foreign”. Together with the lab of Dr. Wei Cheng in the University of Michigan, we are studying a novel set of viral-like model antigens to understand at the cellular and molecular level how B cells perceive antigen affinity and density on a virus.
Regulation and selection of self-reactivity in the B cell repertoire
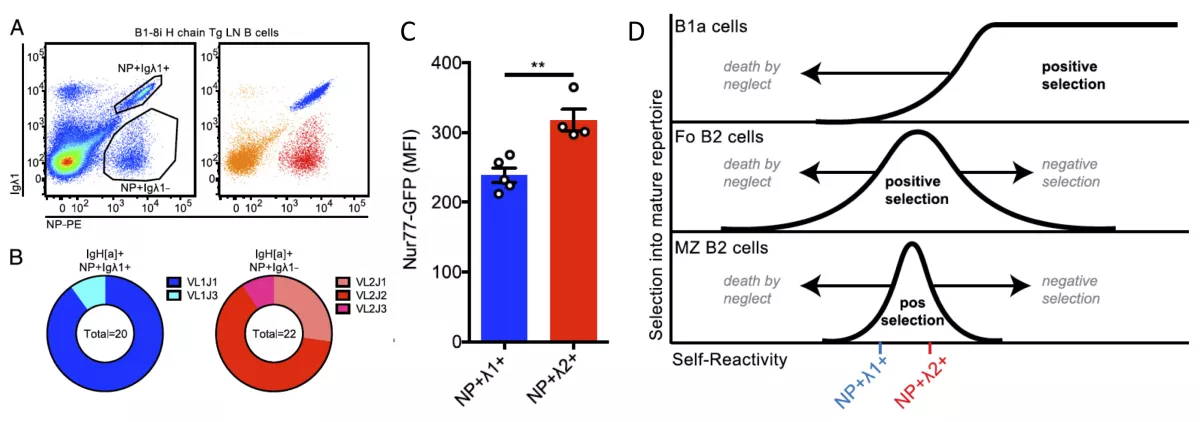
We have exploited a reporter of antigen receptor signaling (Nur77-eGFP) to show that its expression identifies self-reactive B cells in vivo. This enables us to study the phenotype, functional capacities, and genetic program that regulates these cells. It has allowed us to study both positive and negative selection of self-reactivity into the mature naïve B cell repertoire.
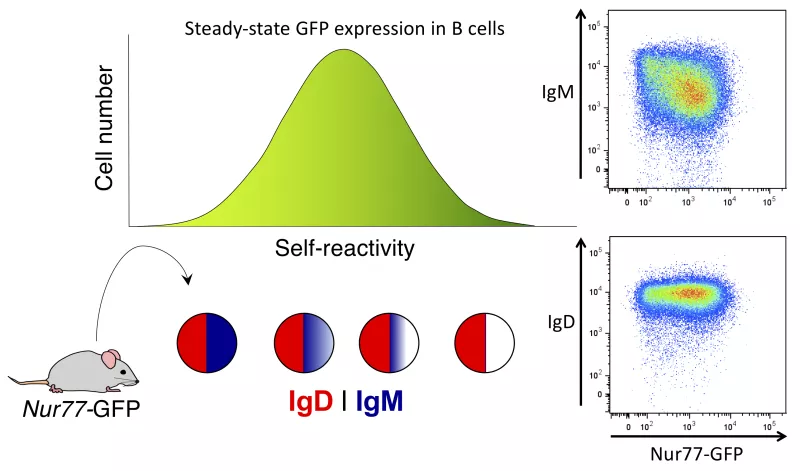
Regulation of B cell tolerance by IgM and IgD antigen receptors
Since IgM and IgD BCRs differ in their Fc region, but not in their antigen-binding domain, it has been unclear why two different BCRs are co-expressed by naive mature B cells. Perhaps the most notable phenotypic and functional characteristic of self-reactive naive B cells that express high levels of Nur77-eGFP reporter is progressive downregulation of surface IgM BCR. By contrast, surface IgD BCR expression is unaltered. Our lab asked how downregulation of IgM in the face of persistent high IgD expression might impose tolerance on auto-reactive B cells. To address this question, we have introduced the Nur77-eGFP reporter of endogenous antigen-dependent signaling onto genetic backgrounds lacking either the IgM or the IgD BCRs.